——————————————————————————————————————————————————————
Story #29 of 50 days, 50 Stories Campaign
——————————————————————————————————————————————————————
Matched Unrelated Donors are considered on high priority for vaccination against COVID-19, and are encouraged to proceed with vaccination as early as possible. In principle, there should be appropriate intervals between vaccine administration, G-CSF mobilization and collection.
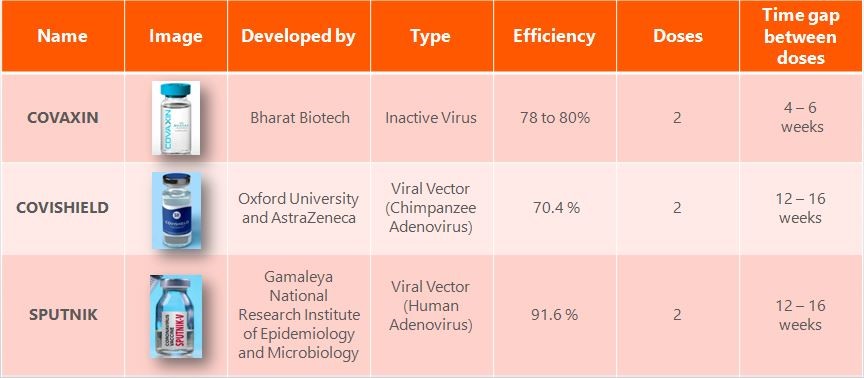
Vaccines available in India:
In January 2021, the central drugs standard control organization (CDSCO), India had granted the emergency-use authorization to two vaccines namely, Covishield (live vaccine, Oxford-AstraZeneca, United Kingdom, being manufactured by the Serum Institute of India, Pune) and Covaxin (inactivated vaccine, Bharat Biotech, India). The major difference between these two vaccines is that the Covishield is a live vaccine while Covaxin is an inactivated vaccine.
Sputnik V also known as Gam-COVID-Vac was the first COVID-19 vaccine to be registered for use in any nation, and it has since been approved in 67 countries. Sputnik V is an adenovirus vaccine, which means that it uses an engineered adenovirus, a family of viruses that generally cause only mild illness, as a delivery mechanism for inserting the genetic code for the SARS-CoV-2 spike protein into human cells. It is similar to the Oxford–AstraZeneca and Johnson & Johnson vaccines. Below table explains the vaccines type available in India,
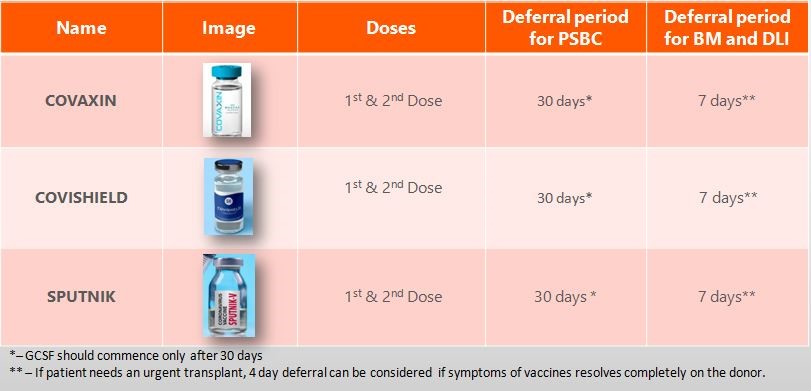
Recommendations on donor deferral for PBSC/BM/DLI:
Why Deferral period is important?
The rapid implementation of COVID-19 vaccines makes it very important to compile comprehensive and accurate side effect data, and thus far these vaccines show a generally higher frequency, severity, and duration of common side effects compared to most other vaccines. An appropriate interval should be scheduled between vaccination and donor workup in order to minimize the risk of side effects combining or being attributed. These side effects are similar to that of GCSF.
There are reports emerging of a rare vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) occurring after the vaccine administration. While published evidence for adverse immune interaction with G-CSF is so far lacking, it would be prudent to avoid G-CSF within the most likely period of VIPIT onset following vaccination. Hence different registries in different countries follow different deferral based on the vaccines used.
*Disclaimer: The deferral period may change in the future as and when there are new studies and evidence which come related to vaccination.
COVID-19 vaccination after donation:
COVID-19 vaccine should not be given until the donor has fully recovered from PBSC, BM or DLI donation. COVID-19 vaccines that use a virus vector could incur VIPIT risk following G-CSF for an unknown period, and where available it would be prudent to use an alternative vaccine that does not use a virus vectors.
Donation deferment for vaccinated donors:
- If Vaccine is not taken – PBSC / BM donation can be done after a negative report is received
- If the vaccine has been taken – then deferment of PBSC for 30 days after vaccination. For BM, deferment of at least 1 week after vaccine side effects resolve. A negative report of COVID is required as per defined protocols
- If the donor has persistent side effects of the vaccine, then a doctor’s clearance would be required before any work-up can be planned.
*Disclaimer: The deferral period may change in the future as and when there are new studies
About DATRI:
DATRI is a Not-for-Profit organization founded in 2009 with a mission to save lives of those suffering from life-threatening Fatal blood disorders like Blood Cancer, Thalassemia, Leukemia, Aplastic Anemia, Sickle Cell Anaemia, etc.
DATRI is registered with the Government of India as a Section 8 organization, and all monetary contributions towards DATRI are subject to 80G exemptions

About the Author:
Jayashree Shankar – a double master’s graduate in Biotechnology, has in-depth knowledge of Stem cells, Human Leukocyte Antigens (HLA) which plays a critical role in Human Blood Stem cell collection and Transplant process. She is associated with DATRI for more than 7 years. She has been working as a Transplant Center Manager and coordinates closely with various National Hospitals. Been with DATRI from very initial time, she has contributed in many developments of DATRI with related to process, forms, data and many more. She is so passionate in achieving DATRI’s Vision.